Which Of The Following Is A Form Of Direct Dna Repair?
Although genetic variation is important for evolution, the survival of the individual demands genetic stability. Maintaining genetic stability requires not only an extremely accurate mechanism for replicating Dna, but also mechanisms for repairing the many adventitious lesions that occur continually in DNA. Well-nigh such spontaneous changes in DNA are temporary because they are immediately corrected by a ready of processes that are collectively called Deoxyribonucleic acid repair. Of the thousands of random changes created every twenty-four hour period in the Deoxyribonucleic acid of a human cell by heat, metabolic accidents, radiations of diverse sorts, and exposure to substances in the environment, only a few accrue every bit mutations in the DNA sequence. Nosotros at present know that fewer than one in yard accidental base changes in DNA results in a permanent mutation; the remainder are eliminated with remarkable efficiency by Deoxyribonucleic acid repair.
The importance of Deoxyribonucleic acid repair is evident from the large investment that cells make in Dna repair enzymes. For example, analysis of the genomes of bacteria and yeasts has revealed that several percent of the coding capacity of these organisms is devoted solely to Deoxyribonucleic acid repair functions. The importance of DNA repair is too demonstrated by the increased rate of mutation that follows the inactivation of a Dna repair gene. Many Dna repair pathways and the genes that encode them—which we now know operate in a wide diverseness of organisms, including humans—were originally identified in bacteria by the isolation and characterization of mutants that displayed an increased mutation charge per unit or an increased sensitivity to DNA-dissentious agents.
Contempo studies of the consequences of a diminished capacity for DNA repair in humans have linked a diversity of human diseases with decreased repair (Table 5-2). Thus, we saw previously that defects in a human gene that normally functions to repair the mismatched base pairs in Deoxyribonucleic acid resulting from replication errors can lead to an inherited predisposition to certain cancers, reflecting an increased mutation rate. In another human disease, xeroderma pigmentosum (XP), the afflicted individuals take an extreme sensitivity to ultraviolet radiation because they are unable to repair certain Dna photoproducts. This repair defect results in an increased mutation rate that leads to serious skin lesions and an increased susceptibility to certain cancers.
Table 5-2
Inherited Syndromes with Defects in DNA Repair.
Without Deoxyribonucleic acid Repair, Spontaneous DNA Harm Would Quickly Modify Dna Sequences
Although Dna is a highly stable material, equally required for the storage of genetic information, it is a complex organic molecule that is susceptible, fifty-fifty nether normal cellular conditions, to spontaneous changes that would lead to mutations if left unrepaired (Figure five-46). DNA undergoes major changes as a consequence of thermal fluctuations: for example, about 5000 purine bases (adenine and guanine) are lost every day from the DNA of each man prison cell because their Northward-glycosyl linkages to deoxyribose hydrolyze, a spontaneous reaction called depurination. Similarly, a spontaneous deamination of cytosine to uracil in DNA occurs at a rate of virtually 100 bases per prison cell per day (Figure 5-47). Dna bases are also occasionally damaged by an run across with reactive metabolites (including reactive forms of oxygen) or environmental chemicals. Besides, ultraviolet radiation from the sun can produce a covalent linkage betwixt two adjacent pyrimidine bases in Dna to form, for example, thymine dimers (Effigy 5-48). If left uncorrected when the Deoxyribonucleic acid is replicated, most of these changes would exist expected to pb either to the deletion of i or more base pairs or to a base-pair substitution in the daughter DNA chain (Figure 5-49). The mutations would and then be propagated throughout subsequent jail cell generations as the DNA is replicated. Such a high charge per unit of random changes in the Dna sequence would accept disastrous consequences for an organism.

Effigy five-46
A summary of spontaneous alterations likely to require Deoxyribonucleic acid repair. The sites on each nucleotide that are known to exist modified past spontaneous oxidative harm (red arrows), hydrolytic assault (blue arrows), and uncontrolled methylation past the methyl grouping (more than...)
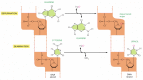
Figure 5-47
Depurination and deamination. These two reactions are the most frequent spontaneous chemical reactions known to create serious Dna impairment in cells. Depurination can release guanine (shown hither), likewise equally adenine, from Deoxyribonucleic acid. The major blazon of deamination (more...)
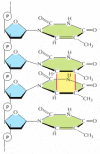
Figure 5-48
The thymine dimer. This blazon of damage is introduced into DNA in cells that are exposed to ultraviolet irradiation (as in sunlight). A similar dimer will form between any 2 neighboring pyrimidine bases (C or T residues) in Dna.
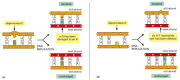
Figure 5-49
How chemical modifications of nucleotides produce mutations. (A) Deamination of cytosine, if uncorrected, results in the substitution of one base for another when the Deoxyribonucleic acid is replicated. Equally shown in Figure v-47, deamination of cytosine produces uracil. (more...)
The DNA Double Helix Is Readily Repaired
The double-helical construction of DNA is ideally suited for repair because it carries two separate copies of all the genetic information—one in each of its two strands. Thus, when i strand is damaged, the complementary strand retains an intact copy of the same data, and this copy is by and large used to restore the correct nucleotide sequences to the damaged strand.
An indication of the importance of a double-stranded helix to the safe storage of genetic information is that all cells use it; simply a few pocket-size viruses utilize single-stranded DNA or RNA every bit their genetic material. The types of repair processes described in this section cannot operate on such nucleic acids, and the chance of a permanent nucleotide change occurring in these unmarried-stranded genomes of viruses is thus very high. It seems that only organisms with tiny genomes can afford to encode their genetic data in whatsoever molecule other than a DNA double helix.
Each cell contains multiple Dna repair systems, each with its own enzymes and preferences for the type of damage recognized. Every bit we meet in the rest of this section, most of these systems use the undamaged strand of the double helix as a template to repair the damaged strand.
Dna Damage Tin Exist Removed past More Than I Pathway
There are multiple pathways for DNA repair, using different enzymes that act upon different kinds of lesions. Two of the most common pathways are shown in Figure 5-50. In both, the damage is excised, the original Dna sequence is restored by a DNA polymerase that uses the undamaged strand as its template, and the remaining suspension in the double helix is sealed by DNA ligase (see Figure 5-14).
Effigy five-50
A comparison of ii major DNA repair pathways. (A) Base excision repair. This pathway starts with a Deoxyribonucleic acid glycosylase. Here the enzyme uracil DNA glycosylase removes an accidentally deaminated cytosine in Deoxyribonucleic acid. After the action of this glycosylase (or another (more...)
The two pathways differ in the way in which the impairment is removed from Deoxyribonucleic acid. The get-go pathway, called base excision repair, involves a bombardment of enzymes chosen Dna glycosylases, each of which can recognize a specific blazon of altered base of operations in Deoxyribonucleic acid and catalyze its hydrolytic removal. There are at to the lowest degree six types of these enzymes, including those that remove deaminated Cs, deaminated As, different types of alkylated or oxidized bases, bases with opened rings, and bases in which a carbon–carbon double bail has been accidentally converted to a carbon–carbon single bail.
As an example of the general machinery of base of operations excision repair, the removal of a deaminated C past uracil Deoxyribonucleic acid glycosylase is shown in Figure five-50A. How is the altered base of operations detected within the context of the double helix? A key footstep is an enzyme-mediated "flipping-out" of the altered nucleotide from the helix, which allows the enzyme to probe all faces of the base for damage (Figure 5-51). It is thought that DNA glycosylases travel along Deoxyribonucleic acid using base-flipping to evaluate the status of each base pair. One time a damaged base is recognized, the Deoxyribonucleic acid glycosylase reaction creates a deoxyribose sugar that lacks its base. This "missing molar" is recognized past an enzyme chosen AP endonuclease, which cuts the phosphodiester backbone, and the damage is and so removed and repaired (run into Figure 5-50A). Depurination, which is by far the most frequent blazon of damage suffered by Dna, likewise leaves a deoxyribose saccharide with a missing base. Depurinations are directly repaired beginning with AP endonuclease, following the lesser half of the pathway in Effigy 5-50A.

Figure 5-51
The recognition of an unusual nucleotide in DNA by base of operations-flipping. The DNA glycosylase family unit of enzymes recognizes specific bases in the conformation shown. Each of these enzymes cleaves the glycosyl bond that connects a particular recognized base of operations (yellowish) (more...)
The second major repair pathway is chosen nucleotide excision repair. This mechanism can repair the damage caused by near any large change in the structure of the Deoxyribonucleic acid double helix. Such "bulky lesions" include those created by the covalent reaction of Dna bases with large hydrocarbons (such equally the carcinogen benzopyrene), too every bit the various pyrimidine dimers (T-T, T-C, and C-C) caused past sunlight. In this pathway, a big multienzyme circuitous scans the DNA for a baloney in the double helix, rather than for a specific base change. In one case a beefy lesion has been found, the phosphodiester backbone of the abnormal strand is cleaved on both sides of the distortion, and an oligonucleotide containing the lesion is peeled away from the Dna double helix by a Dna helicase enzyme. The large gap produced in the Dna helix is then repaired by DNA polymerase and DNA ligase (Effigy v-50B).
The Chemistry of the Dna Bases Facilitates Impairment Detection
The DNA double helix seems to be optimally constructed for repair. As noted above, it contains a backup copy of the genetic information, so that if one strand is damaged, the other undamaged strand can be used equally a template for repair. The nature of the bases as well facilitates the stardom between undamaged and damaged bases. Thus, every possible deamination event in Dna yields an unnatural base, which can therefore exist directly recognized and removed by a specific Deoxyribonucleic acid glycosylase. Hypoxanthine, for example, is the simplest purine base capable of pairing specifically with C, just hypoxanthine is the direct deamination product of A (Figure five-52A). The improver of a second amino grouping to hypoxanthine produces Grand, which cannot be formed from A past spontaneous deamination, and whose deamination production is likewise unique.
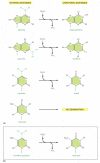
Figure 5-52
The deamination of DNA nucleotides. In each case the oxygen atom that is added in this reaction with water is colored ruddy. (A) The spontaneous deamination products of A and G are recognizable every bit unnatural when they occur in Deoxyribonucleic acid and thus are readily recognized (more than...)
As discussed in Chapter 6, RNA is idea, on an evolutionary time-scale, to have served as the genetic material before Dna, and it seems probable that the genetic code was initially carried in the four nucleotides A, C, Yard, and U. This raises the question of why the U in RNA was replaced in DNA past T (which is 5-methyl U). We accept seen that the spontaneous deamination of C converts information technology to U, but that this event is rendered relatively harmless by uracil Dna glycosylase. However, if Dna independent U as a natural base of operations, the repair system would exist unable to distinguish a deaminated C from a naturally occuring U.
A special situation occurs in vertebrate DNA, in which selected C nucleotides are methylated at specific C-Chiliad sequences that are associated with inactive genes (discussed in Chapter 7). The accidental deamination of these methylated C nucleotides produces the natural nucleotide T (Figure 5-52B) in a mismatched base pair with a G on the opposite Deoxyribonucleic acid strand. To help in repairing deaminated methylated C nucleotides, a special Deoxyribonucleic acid glycosylase recognizes a mismatched base pair involving T in the sequence T-One thousand and removes the T. This DNA repair mechanism must exist relatively ineffective, notwithstanding, because methylated C nucleotides are common sites for mutations in vertebrate Dna. It is hitting that, fifty-fifty though only almost 3% of the C nucleotides in homo DNA are methylated, mutations in these methylated nucleotides account for nigh one-third of the unmarried-base mutations that have been observed in inherited human diseases.
Double-Strand Breaks are Efficiently Repaired
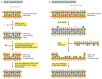
A potentially dangerous type of DNA damage occurs when both strands of the double helix are broken, leaving no intact template strand for repair. Breaks of this blazon are caused past ionizing radiation, oxidizing agents, replication errors, and certain metabolic products in the cell. If these lesions were left unrepaired, they would rapidly lead to the breakdown of chromosomes into smaller fragments. However, two singled-out mechanisms have evolved to improve the potential damage. The simplest to understand is nonhomologous end-joining, in which the broken ends are juxtaposed and rejoined by DNA ligation, generally with the loss of one or more nucleotides at the site of joining (Effigy 5-53A). This end-joining mechanism, which tin exist viewed as an emergency solution to the repair of double-strand breaks, is a common outcome in mammalian cells. Although a change in the DNA sequence (a mutation) results at the site of breakage, so little of the mammalian genome codes for proteins that this mechanism is apparently an acceptable solution to the problem of keeping chromosomes intact. As previously discussed, the specialized structure of telomeres prevents the ends of chromosomes from beingness mistaken for cleaved DNA, thereby preserving natural DNA ends.
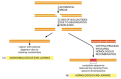
Figure v-53
Ii different types of end-joining for repairing double-strand breaks. (A) Nonhomologous terminate-joining alters the original DNA sequence when repairing broken chromosomes. These alterations can be either deletions (as shown) or curt insertions. (B) Homologous (more...)
An even more than effective type of double-strand break repair exploits the fact that cells that are diploid contain ii copies of each double helix. In this second repair pathway, chosen homologous end-joining, general recombination mechanisms are chosen into play that transfer nucleotide sequence information from the intact DNA double helix to the site of the double-strand suspension in the broken helix. This blazon of reaction requires special recombination proteins that recognize areas of Dna sequence matching betwixt the ii chromosomes and bring them together. A Deoxyribonucleic acid replication process then uses the undamaged chromosome as the template for transferring genetic data to the broken chromosome, repairing it with no change in the DNA sequence (Figure 5-53B). In cells that have replicated their DNA but not nonetheless divided, this type of Deoxyribonucleic acid repair can readily take place between the 2 sister Deoxyribonucleic acid molecules in each chromosome; in this case, in that location is no need for the cleaved ends to find the matching Deoxyribonucleic acid sequence in the homologous chromosome. The molecular details of the homologous end-joining reaction are considered later in this chapter because they require a general understanding of the way in which cells comport their genetic recombination events. Although present in humans, this type of DNA double-strand pause repair predominates in bacteria, yeasts, and Drosophila—all organisms in which lilliputian nonhomologous DNA end-joining is observed.
Cells Can Produce DNA Repair Enzymes in Response to Deoxyribonucleic acid Damage
Cells have evolved many mechanisms that help them survive in an unpredictably hazardous earth. Often an farthermost alter in a prison cell's environment activates the expression of a prepare of genes whose protein products protect the cell from the deleterious effects of this change. One such mechanism shared by all cells is the estrus-daze response, which is evoked by the exposure of cells to unusually loftier temperatures. The induced "estrus-shock proteins" include some that help stabilize and repair partly denatured jail cell proteins, equally discussed in Chapter vi.
Cells likewise have mechanisms that elevate the levels of Dna repair enzymes, as an emergency response to severe Dna damage. The best-studied example is the so-called SOS response in Eastward. coli. In this bacterium, whatsoever cake to Dna replication acquired past Deoxyribonucleic acid damage produces a signal that induces an increase in the transcription of more than 15 genes, many of which code for proteins that function in Dna repair. The bespeak (thought to exist an excess of single-stranded DNA) kickoff activates the E. coli RecA protein (come across Effigy five-58), so that it destroys a gene regulatory protein that normally represses the transcription of a large set of SOS response genes.
Studies of mutant bacteria deficient in dissimilar parts of the SOS response demonstrate that the newly synthesized proteins take ii effects. Beginning, every bit would be expected, the induction of these additional Deoxyribonucleic acid repair enzymes increases prison cell survival after Dna damage. Second, several of the induced proteins transiently increase the mutation rate by increasing the number of errors made in copying Deoxyribonucleic acid sequences. The errors are caused by the product of low-fidelity DNA polymerases that tin can efficiently use damaged DNA every bit a template for DNA synthesis. While this "error-prone" DNA repair can be harmful to individual bacterial cells, it is presumed to be advantageous in the long term because information technology produces a outburst of genetic variability in the bacterial population that increases the likelihood of a mutant cell arising that is better able to survive in the altered environs.
Human cells contain more than 10 minor DNA polymerases, many of which are specifically called into play, equally a last resort, to copy over unrepaired lesions in the DNA template. These enzymes tin recognize a specific type of Deoxyribonucleic acid damage and add together the nucleotides that restore the initial sequence. Each such polymerase molecule is given a hazard to add together simply one or a few nucleotides, because these enzymes are extremely error-decumbent when they re-create a normal Deoxyribonucleic acid sequence. Although the details of these fascinating reactions are notwithstanding existence worked out, they provide elegant testimony to the intendance with which organisms maintain their DNA sequences.
Dna Damage Delays Progression of the Cell Cycle
Nosotros have simply seen that cells contain multiple enzyme systems that can recognize Dna impairment and promote the repair of these lesions. Considering of the importance of maintaining intact, undamaged Deoxyribonucleic acid from generation to generation, cells have an boosted mechanism that helps them respond to Deoxyribonucleic acid damage: they delay progression of the cell cycle until DNA repair is complete. For example, 1 of the genes expressed in response to the E. coli SOS point is sulA, which encodes an inhibitor of cell division. Thus, when the SOS functions are turned on in response to Dna impairment, a block to jail cell partition extends the time for repair. When Deoxyribonucleic acid repair is consummate, the expression of the SOS genes is repressed, the cell cyle resumes, and the undamaged DNA is segregated to the daughter cells.
Damaged DNA too generates signals that block prison cell-cycle progression in eucaryotes. Equally discussed in detail in Affiliate 17, the orderly progression of the cell cycle is maintained through the use of checkpoints that ensure the completion of ane step earlier the adjacent pace tin begin. At several of these cell-bike checkpoints, the cycle stops if damaged Dna is detected. Thus, in yeast, the presence of DNA damage tin can cake entry into the G1 phase; information technology tin can dull Dna replication in one case begun; and it tin can block the transition from S phase to M phase. The Deoxyribonucleic acid damage results in an increased synthesis of some DNA repair enzymes, and the delays further facilitate repair past providing the time needed for repair to reach completion.
The importance of the special signaling mechanisms that respond to Deoxyribonucleic acid damage is indicated by the phenotype of humans who are born with defects in the factor that encodes the ATM protein, a big poly peptide kinase. These individuals have the illness clutter–telangiectasia (AT), whose symptoms include neurodegeneration, a predisposition to cancer, and genome instability. In both humans and yeasts, the ATM protein is needed to generate the initial intracellular signals that produce a response to oxygen-inflicted DNA damage, and individual organisms with defects in this protein are hypersensitive to agents that cause such damage, such as ionizing radiation.
Summary
Genetic data tin can be stored stably in Dna sequences only because a big set up of Dna repair enzymes continuously browse the Deoxyribonucleic acid and supplant any damaged nucleotides. Virtually types of Dna repair depend on the presence of a carve up re-create of the genetic information in each of the ii strands of the Dna double helix. An accidental lesion on i strand tin therefore be cut out past a repair enzyme and a corrected strand resynthesized by reference to the data in the undamaged strand.
Nearly of the damage to Deoxyribonucleic acid bases is excised by 1 of 2 major Deoxyribonucleic acid repair pathways. In base excision repair, the altered base is removed by a DNA glycosylase enzyme, followed by excision of the resulting sugar phosphate. In nucleotide excision repair, a small section of the Deoxyribonucleic acid strand surrounding the damage is removed from the Dna double helix as an oligonucleotide. In both cases, the gap left in the DNA helix is filled in by the sequential action of Deoxyribonucleic acid polymerase and Deoxyribonucleic acid ligase, using the undamaged Deoxyribonucleic acid strand as the template.
Other critical repair systems—based on either nonhomologous or homologous stop-joining mechanisms—reseal the accidental double-strand breaks that occur in the Dna helix. In almost cells, an elevated level of DNA damage causes both an increased synthesis of repair enzymes and a delay in the cell bike. Both factors assist to ensure that DNA damage is repaired earlier a cell divides.


Which Of The Following Is A Form Of Direct Dna Repair?,
Source: https://www.ncbi.nlm.nih.gov/books/NBK26879/
Posted by: mccrayfourgaver99.blogspot.com


0 Response to "Which Of The Following Is A Form Of Direct Dna Repair?"
Post a Comment